Meet four scientists using different types of light microscopy for virology research
The MRC-University of Glasgow Centre for Virus Research (CVR) was established in 2010 and represents the UK’s largest grouping of human and veterinary virologists.
The CVR is embedded within the Institute of Infection, Immunity and Inflammation at the University of Glasgow, which provides excellent research opportunities to investigate virus-host interactions and immune response to virus infection. A defining feature of the CVR is its breadth of expertise ranging from molecular virology to in vivo pathogenesis, virus-cell interactions, viral immunology, viral ecology, viral oncology, clinical and veterinary virology, viral diagnostics, virus epidemiology, mathematical modelling, virus genomics & bioinformatics.
We profiled four scientists who use light microscopy to highlight some of the research being performed at the CVR.
Studying viruses with light sheet microscopy
Dr. Steph Rainey is a Research Fellow working within Professor Steven Sinkins’ Group.
She works on trying to understand how the bacterium Wolbachia blocks the replication of clinically important arboviruses, such as dengue. The primary vector for dengue, Aedes aegypti (mosquito), does not naturally harbor Wolbachia, which is found in ~66% of insects. However, the Sinkins’ group is able to transinfect the bacterium into the mosquito and have shown a 80% reduction in dengue cases in their Malaysian release site, compared to control sites.

Dr. Steph Rainey in front of her mosquito colonies
To understand how Wolbachia blocks viruses, Dr. Rainey and the group uses a range of techniques to look at what is going on at a cellular level. They infect cells and mosquitoes with different arboviruses (arthropod/insect borne viruses) and then use microscopy to see how Wolbachia affects the replication complexes, where the virus multiplies and how both affect the structures inside the cell. To do this they use both confocal and light sheet microscopy. They use live samples, fixed samples, and cells.
One of the things they have found is that cholesterol trafficking is important for blocking viruses when Wolbachia is present. They also use fluorescence in situ hybridization to look at density and location of the Wolbachia within tissues and cells.
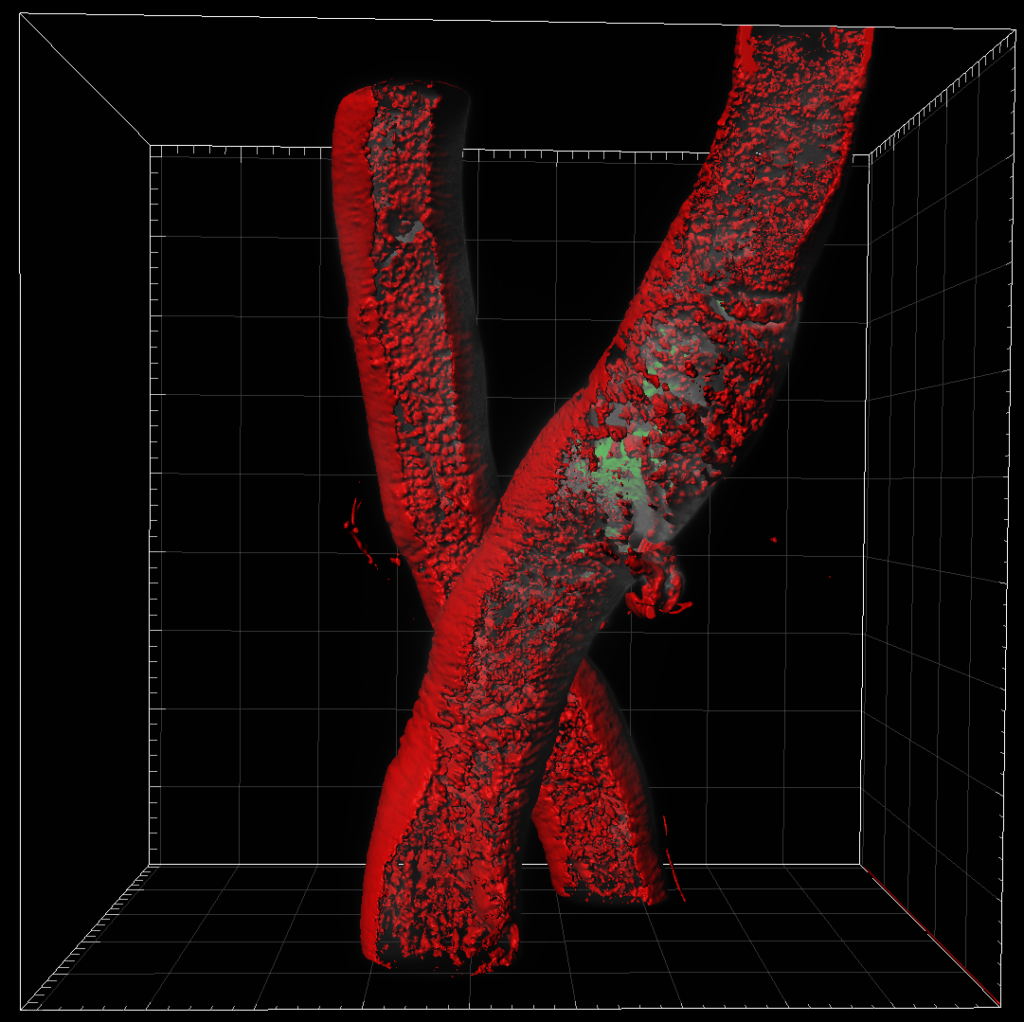
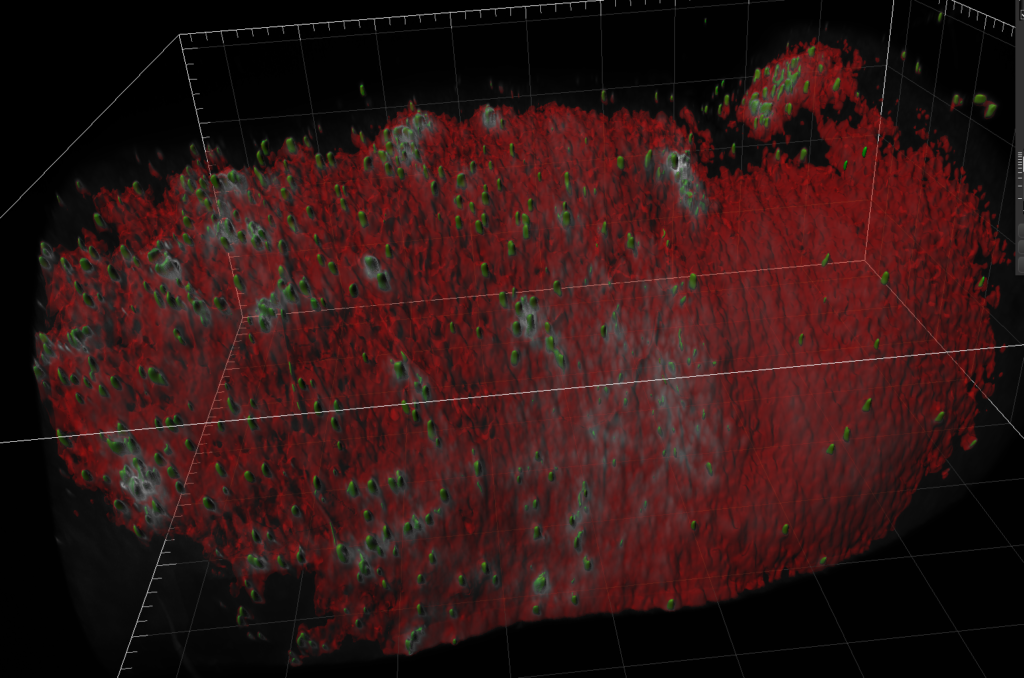
Left Image: Aedes aegypti mosquito Malpighian tubule. We can see the clear tube-like structure of the tubules. Right image: Aedes aegypti mosquito midguts and lipid content. The depth of scan obtained using light sheet microscopy gives us an image that could not be obtained on other platforms and allows us to clearly visualize the lipids. Both images were obtained using a ZEISS light sheet microscope and rendered in Imaris. Image credit: Stephanie Rainey, Maria Vittoria Mancini & Colin Loney
When asked why she likes using light sheet microscopy:
There is nothing like a good picture to tell a story. A lot of the time science is about numbers and graphs. I have a very visual brain so being able to see what is going on helps a lot.
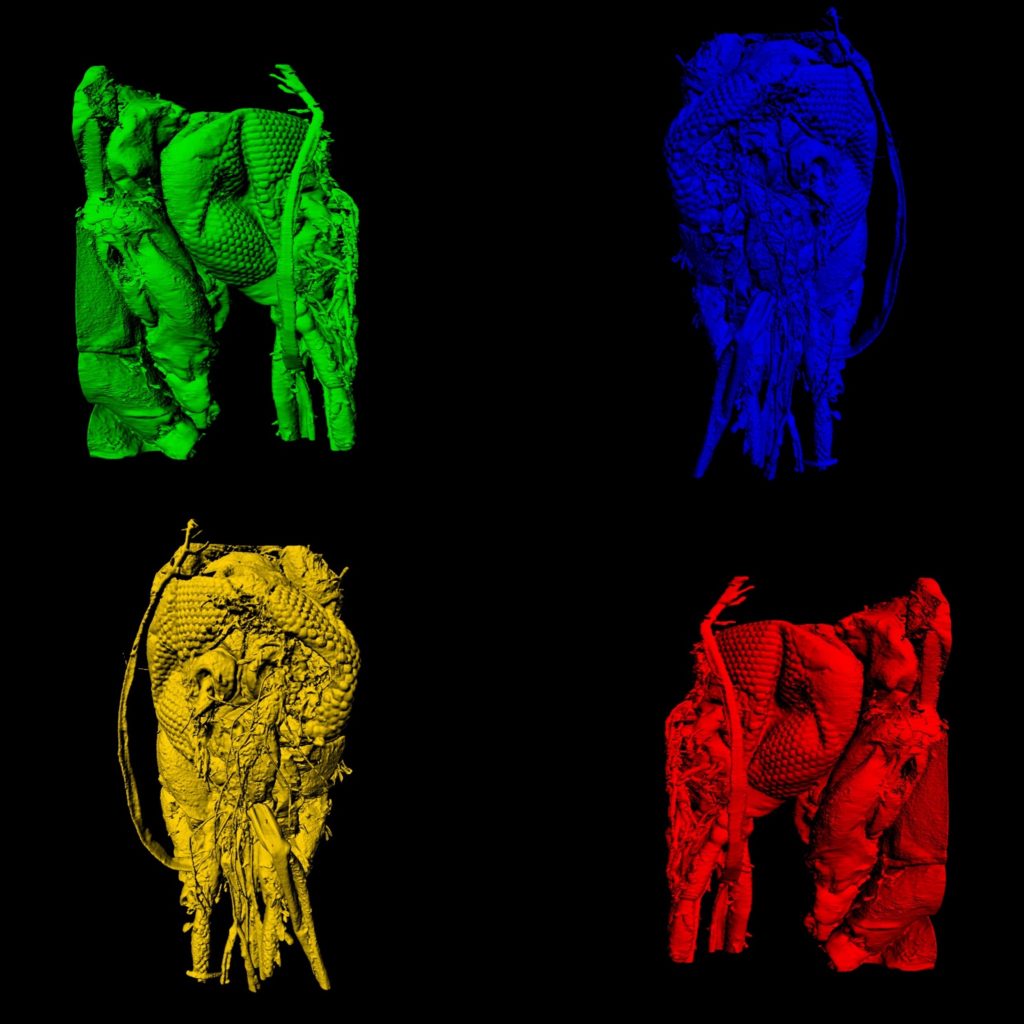
Four sides of a mosquito head imaged using a ZEISS light sheet microscope and rendered in Imaris. We are able to see in amazing detail the anatomy of the head, mouth parts and eyes of the mosquito. Image credit: Stephanie Rainey, Maria Vittoria Mancini & Colin Loney
Superresolution imaging to better understand herpes simplex virus-1 (HSV-1)
Dr. Mila Collados Rodriguez is a postdoctoral researcher working within Dr. Chris Boutell’s Group. Currently, she aims to determine how molecular components of promyelocytic leukaemia-nuclear bodies (PML-NBs) drive the repression of the herpes simplex virus-1 (HSV-1) genome.
Dr. Collados Rodriguez uses the Airyscan detector of a ZEISS confocal microscope for superresolution imaging.
Cells are seeded onto cover slips which are either mock treated or infected with labelled HSV-1. At different time-points, cells are permeabilized, fixed and then conjugated with a fluorophore (click chemistry) which allows visualization of the labelled viral DNA. Immunofluorescence on PML-NB components is also performed to investigate the spatiotemporal contribution of PML-NB molecules in restricting HSV-1 infection at a single viral genome resolution.
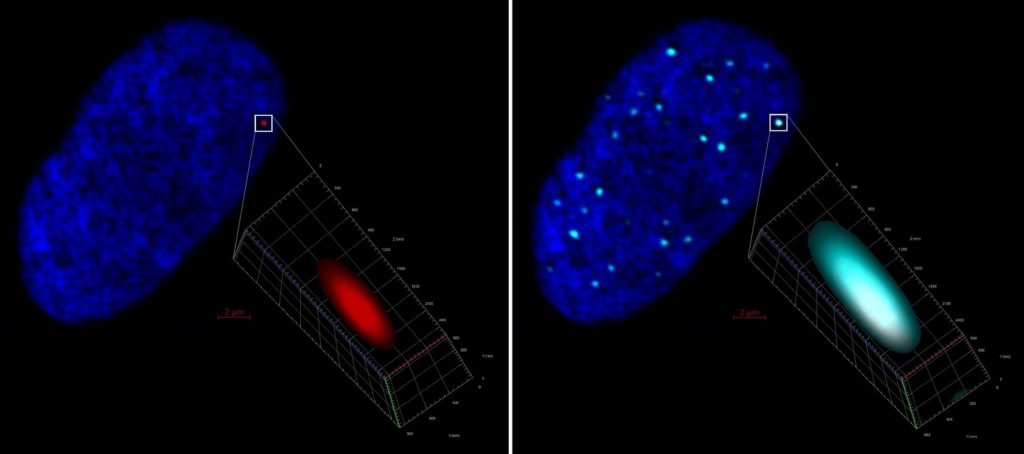

Left, ZEISS LSM 880 Airyscan 2D projection of a human fibroblast cell nucleus (stained with DAPI, blue) harboring a click chemistry red labelled HSV-1 genome further detailed in 3D. Right, same image evidencing colocalization and entrapment of the HSV-1 genome by PML (cyan) immunostaining. Mila Collados Rodríguez, CVR-University of Glasgow, UK
Cell nucleus containing 2 HSV-1 genomes (labelled in red) entrapped by PML (in cyan) imaged in 3D with ZEISS LSM 880 with Airyscan and processed with IMARIS software. Mila Collados Rodríguez, CVR-University of Glasgow, UK.
Using live cell imaging to visualize coinfection of viruses
Joanne Haney is a PhD student working within Dr. Pablo Murcia’s Group. She studies virus interactions during co-infection between common respiratory viruses. She is interested in the dynamics of infection when two viruses are replicating in the same population of cells. She compares the phenotypes of infection between each virus replicating alone, and in the presence of one another to identify differences that may highlight virus-virus interactions.
Live cell imaging is a huge part of her research.

She is able to observe how viral infections spread throughout a population of cells and what effect this has on cell survival and cell movement. It also allowed her to track individual cells to determine how the infection status of that cell (infected with one or both viruses) is related to the viral induced outcome.
Human lung cells infected with two seasonal respiratory viruses that have been tagged with fluorescent proteins. Cell nuclei were stained (blue) to allow them to be easily tracked and cells were imaged over a time course to capture the dynamics of infection and viral spread.
Single cell analysis using laser microdissection to better understand hepatitis C virus

Dr. Carol Leitch is a Research Fellow working within Professor John McLauchlan’s Group. She researches how hepatitis C virus (HCV) affects liver cells (hepatocytes) by comparing the transcriptomes of individual infected and uninfected cells. The end goal of her research is to be able to describe the effect of HCV on both infected and uninfected hepatocytes spatially at the single cell level.
Dr. Leitch uses a laser microdissection microscope, which is extremely accurate, to excise single cells from liver biopsy specimens. To prepare the samples, she uses a cryomicrotome to section fresh-frozen liver needle biopsies. The sections are then fixed in formaldehyde and RNA-FISH is performed with multiple probes against the HCV RNA genome to identify infected cells.
Read select publications from the CVR:
- The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLOS Pathogens
- Distinct temporal roles for the promyelocytic leukaemia (PML) protein in the sequential regulation of intracellular host immunity to HSV-1 infection. PLOS Pathogens
- Perturbed cholesterol and vesicular trafficking associated with dengue blocking in Wolbachia-infected Aedes aegypti cells. Nature Communications