Novel use of expansion microscopy with ZEISS Airyscan technology
Primary cilia are organelles that protrude from the cell surface and function as “cellular antennae” to receive and transduce extracellular signals. They are approximately 3 µm in length and 0.3 µm in diameter, which is below the optical diffraction limit of standard light microscopes.
Junior Associate Professor Yohei Katoh (above, right) and Professor Kazuhisa Nakayama (above, left) at the Department of Physiological Chemistry, Kyoto University (Japan) have developed a novel technique to combine expansion microscopy (ExM) with ZEISS Airyscan superresolution microscopy technology to successfully observe cilia and centrioles with high brightness and resolution. Their findings are published here.
We spoke with Dr. Katoh about their work.
What are the research goals of your lab?
The Nakayama lab has been focusing on biogenesis of primary cilia and the mechanisms underlying regulation of ciliary protein trafficking.
The intraflagellar transport (IFT) machinery powered by kinesin and dynein motor proteins mediates anterograde and retrograde protein trafficking. The IFT machinery is a huge protein complex containing five multisubunit complexes composed of ~40 subunits in total. The Nakayama lab has revealed the architectures of these multisubunit complexes in the IFT machinery, the roles of individual subunits in the complexes, and the recognition mechanisms of cargo proteins by the complexes, and thereby elucidated the molecular mechanisms underlying the regulation of ciliary protein trafficking.
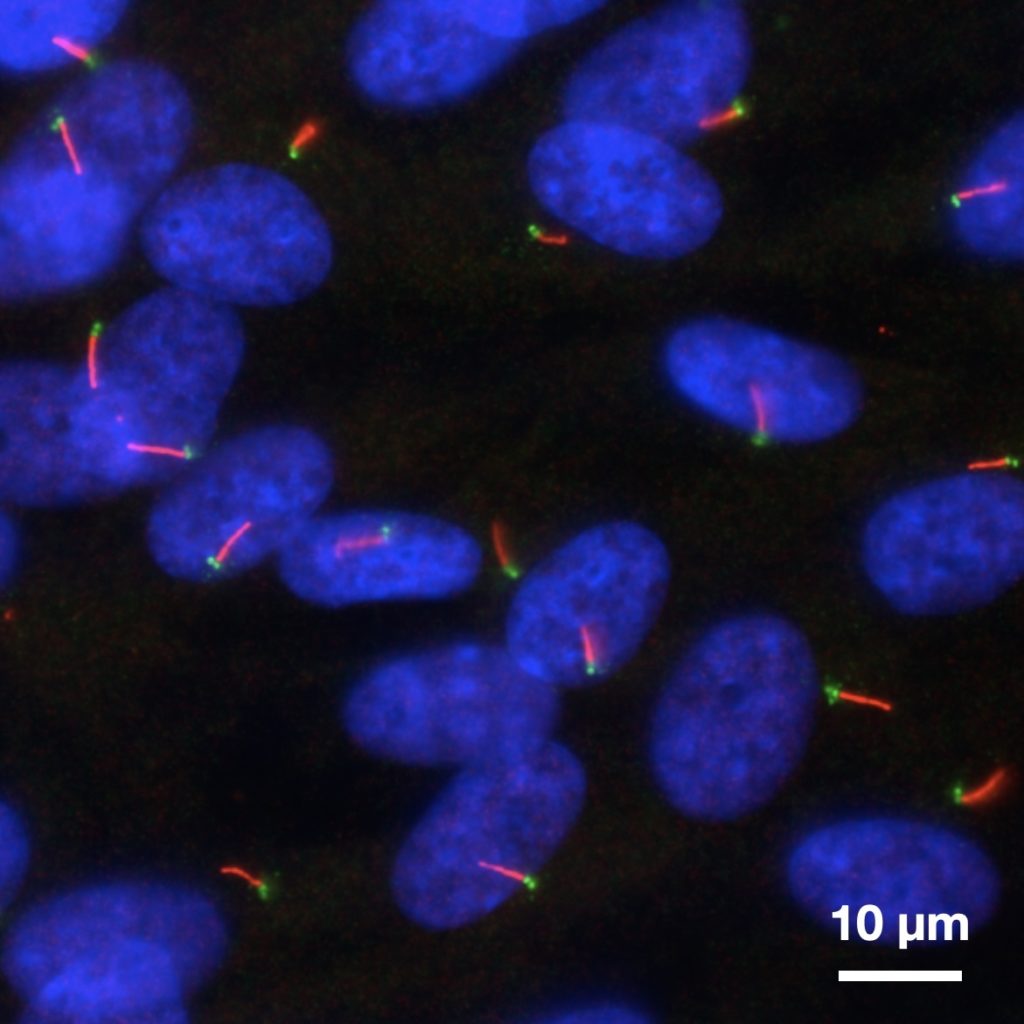
Primary cilia in hTERT-RPE1 cells imaged with conventional microscopy.
Defects in cilia biogenesis and ciliary protein trafficking therefore lead to various hereditary disorders with a broad spectrum of clinical manifestations, generally called the “ciliopathies.” The Nakayama lab also aims to understand the molecular and cellular basis of the ciliopathies.
What gave you the idea to combine Expansion microscopy (ExM) with Airyscan superresolution microscopy?
I had no experience with super-resolution microscopes, such as Airyscan or SIM (structured illumination microscopy), before starting this research. Since there is no super-resolution microscope in our institute as common equipment, I was looking for another method of super-resolution imaging of primary cilia.
When I first read the paper on expansion microscopy (ExM), I was impressed with the innovative idea of using superabsorbent gel to expand the cells. At that time, however, I wondered if this was really possible, and decided to try this innovative strategy myself. I bought some reagents, did some experiments, and was very surprised when I could really make cells expand.
Afterward, at a meeting of the Japanese Biochemical Society held in Osaka, I met Dr. Shuhei Chiba (Osaka City University), who is a skilled microscopist and utilizes super-resolution microscopy to study primary cilia and centrioles. At the meeting, Shuhei was very interested in my ExM images, and Shuhei and I came up with the idea to combine ExM and super-resolution microscopy techniques. I remember that I visited his lab with my ExM samples in the next week.

Dr. Shuhei Chiba. The monitor shows a primary cilium in a post-expansion sample.
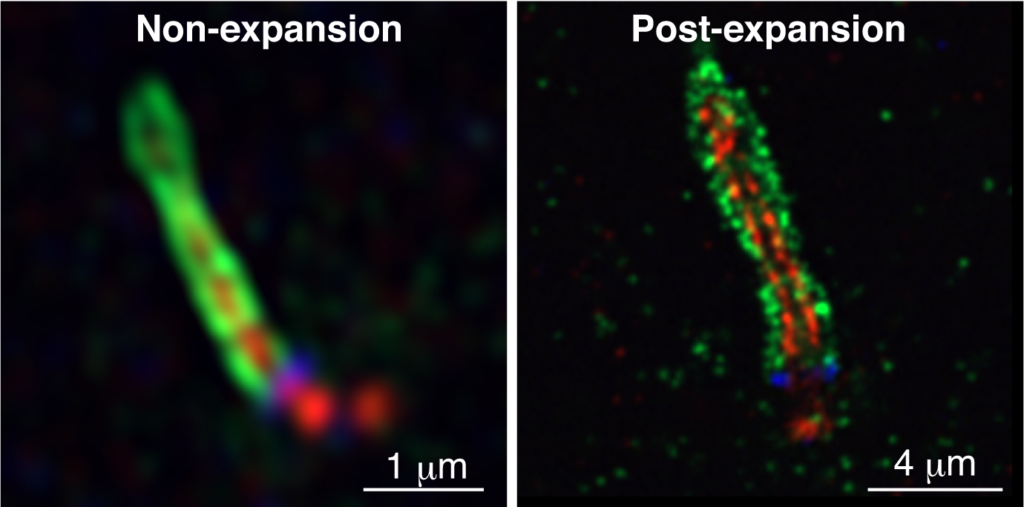

Airyscan analysis of the localization of MyosinVa (green; ciliary vesicle), polyglutamylated tubulin (pGlu-tubulin, red; axonemal microtubules), and CEP164 (blue; distal appendages of mother centriole) in non-expansion (left) and post-expansion (right) cells. The combinatorial use of Airyscan and expansion microscopy dramatically improves the optical resolution and makes multicolor super-resolution imaging more practical.
What was your reaction when you saw the data?
Of course, we were amazed at the image quality. Centrioles, which were only visible as dots under a conventional microscope, appeared to be beautiful nine-fold symmetrical structures when observed by the combinatorial use of ExM and Airyscan.
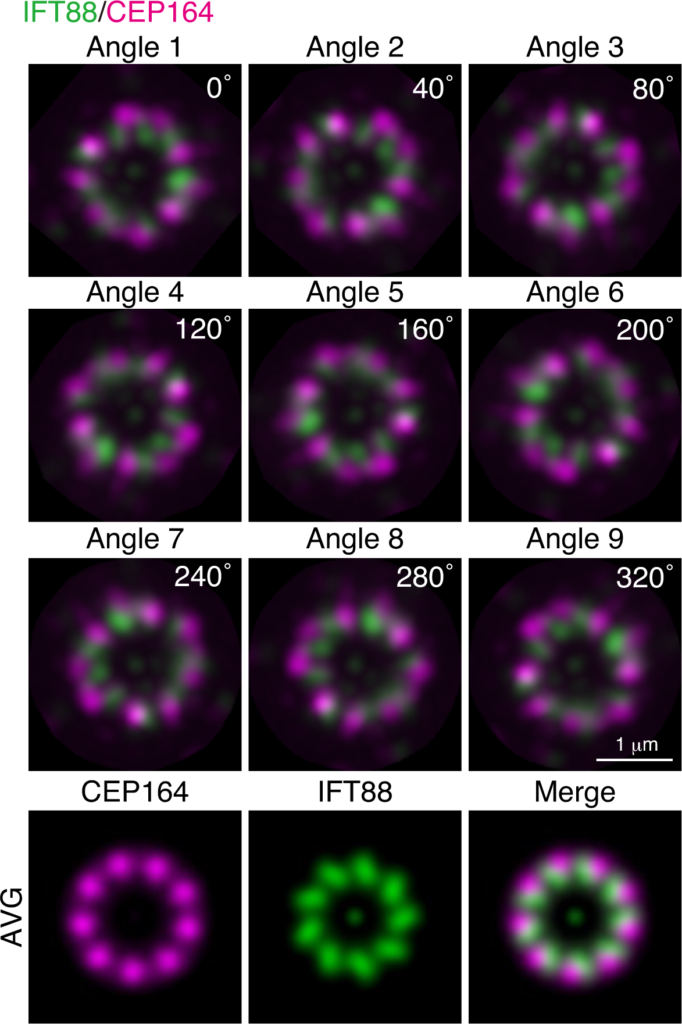

Airyscan image of the localization of intraflagellar transport protein 88 (IFT88), a subunit of anterograde IFT-B complex, and CEP164 in axially oriented mother centriole in post-expanded cells. The averaged maximum projection (AVG) images (bottom three panels) were generated by merging the set of nine-images obtained by rotating maximum intensity projection every 40° into one-stack.
What do you plan to work on next?
The selective entry and exit of proteins into and out of cilia is controlled by the transition zone (TZ) located at the base of cilia. The TZ is composed of more than 20 different proteins, the mutations of which cause ciliopathies with a broad spectrum of clinical manifestations. However, little is known about how the TZ is constructed by these TZ proteins and how it controls the passage of ciliary proteins. Because of the limitations of standard light microscopy to observe the fine structure of the TZ, we are applying ExM and Airyscan to observe the TZ and to elucidate the molecular mechanisms underlying the selective entry and exit of ciliary proteins.
Learn More
Read the full article: Practical method for superresolution imaging of primary cilia and centrioles by expansion microscopy using an amplibody for fluorescence signal amplification Link
Learn about Expansion Microscopy
Get more information on Airyscan, a confocal detector which enables superresolution microscopy on new and many legacy ZEISS LSMs.





