Cryo electron microscopy and EDS offer insights on how diatoms efficiently work with inorganic materials
At the Weizmann Institute of Science (Israel), Dr. Assaf Gal works to understand how nature outperforms us. His group studies how living organisms can control the formation and sculpting of inorganic materials using state-of-the-art cryo electron microscopy (cryoEM) approaches with molecular resolution using the ZEISS Crossbeam FIB-SEM.


In his recent publication, Dr. Gal along with Katya Rechav (Staff Scientist, pictured with Dr. Gal on the left) and Dr. Santosh Kumar (former postdoctoral researcher in Dr. Gal’s lab, shown on the right) developed a methodology using cryoEM and energy dispersive X-ray spectroscopy (EDS) to accurately measure intracellular silicon levels in diatoms. We spoke to Dr. Gal to learn more.
Why study diatoms?
The formation and sculpting of inorganic materials is a widespread phenomenon in the natural world – our own bones and teeth are examples. The interesting point here is that in synthetic and industrial applications, manufacturing of these materials requires extreme chemical conditions and the result is usually bulky, unstructured chunks of material. However, organisms know how to take building blocks from the environment at ambient conditions and control the mineralization process such that the nanopatterned morphology of the material is genetically controlled.
We focus on two groups of marine unicellular algae that master such processes, one of which are diatoms. These tiny cells can form an extracellular cell wall that is made from simple materials like silica or calcium carbonate with exquisite morphologies, unequaled by any man-made process.
Scanning electron microscopy (SEM) images of dried silica cell walls of diatoms.
Diatoms are one group of unicellular organisms that can form silica elements within their cells and then exocytose them. One of the challenges for diatoms is that the silica building blocks (soluble molecules of silicic acid) are very dilute in sea water (about 0.1 mM when it takes more than 1 mM to get above saturation levels). There are many questions regarding how diatoms concentrate silicon in their cells, in which organelles, and to what concentrations. All of these questions were out of reach because it was impossible to directly measure the concentration of an inorganic element like silicon in native cellular conditions.
How did you use electron microscopy and EDS to overcome this challenge?
Our breakthrough is that we developed a methodology that uses cryo electron microscopy (cryoEM) and energy dispersive X-ray spectroscopy (EDS) to measure silicon concentrations within cells. The advantage of cryoEM is that the cells are vitrified in their pristine biological state, without any alteration of the cellular composition. Then, we developed a sophisticated procedure to measure the concentration of silicon with EDS, while taking advantage of the 3D information to understand where in the cell we are measuring.
Not only could we provide quantitative measurements of intracellular silicon levels, but we also found out that these cells keep a constant pool of this element in their cells. A pool that they use whenever there is a need to produce more silica.
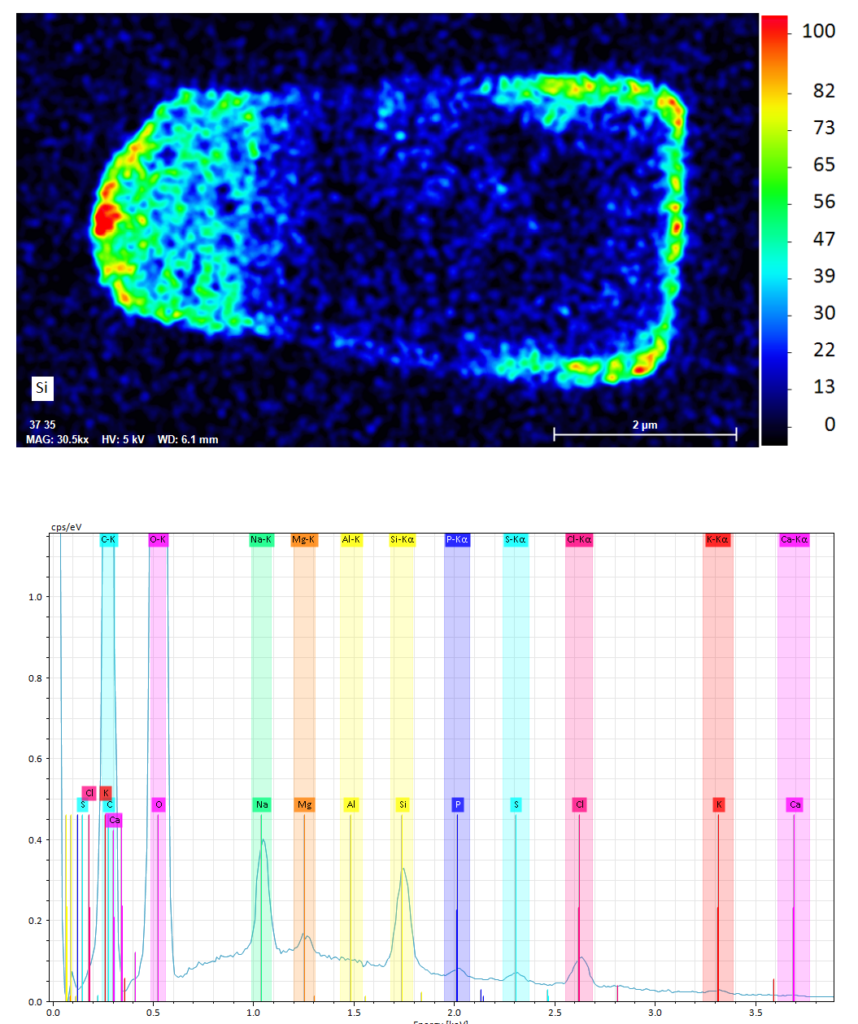
Silicon (Si) EDS map of a cross section through a vitrified diatom cell. Warm colors indicate higher Si concentrations both in the cell wall and the cell interior. The lower panel shows the averaged EDS spectrum of the intracellular volume.
This work is the first to use 3D cryoEM and EDS in a quantitative way to show the distribution and dynamics of intracellular ion pools. This was enabled due to the combination of the new cryoFIB-SEM model of the Crossbeam FIB-SEM with EDS. Only with the ability to simultaneously generate 3D structural data of the cryo preserved samples and the EDS analysis were we able to get these results.
3D rendering of the cellular arrangement of a vitrified diatom cell.
Learn More
Read the full article “Imaging and quantifying homeostatic levels of intracellular silicon in diatoms.” Link
Learn more about the technology used in this article, ZEISS Crossbeam FIB-SEM, and related technology ZEISS Correlative Cryo Workflow solutions.





