Chicago and Vancouver, Canada, June 9, 2022: GE Healthcare is proud to provide cutting-edge molecular imaging solutions that enable and increase access to precision health and theranostics to help improve patient outcomes across care areas, including prostate cancer – the most prevalent cancer in men and the third most prevalent cancer overall[i].
Where most medical therapies are designed with the ‘average’ patient in mind, theranostics brings together diagnoses and treatment in one application, providing a more targeted and personalized therapy than ever before. Clinicians and patients are especially seeing much success with theranostics in prostate cancer – a highly manageable disease, but one that is difficult to treat when diagnosed at a late stage – claiming more than 1.4 million lives annually[ii].
During the COVID-19 pandemic, clinical adoption of theranostics slowed due to a delay in elective procedures and the increased risk posed to its often-immunocompromised patients. However, a surge in demand for theranostics infrastructure[iii] is now anticipated following the U.S. Federal Drug Administration’s (FDA) approval of several new drugs and therapies. This includes the diagnostic tracer Gallium-68 PSMA-11 and therapy drug Lutetium-177 PSMA-617, which are key to applying theranostics in prostate cancer.
To prepare for the creation of dedicated theranostics centers, SNMMI and related international molecular imaging societies recently published a new guide for healthcare systems globally[iii]. Focusing on safety protocols and operational procedures, the guide provides a framework that highlights best practices that can be applied across care areas.
“Nuclear medicine is entering a new age of precision theranostics, in which next-generation alpha- and beta-labeled radiotherapeutics are tailored to individual cancer patients using the latest diagnostic PET radiopharmaceuticals,” explains Dr. Peter Scott, Associate Professor of Radiology, Division Director of Nuclear Medicine, University of Michigan. “With the FDA approval of a new PSMA-agent for treatment of prostate cancer, the future is here. Patients, their families and referring physicians are all demanding access to theranostics, creating an unprecedented demand for higher and higher amounts of radioactive metals. The only way to meet the global need for PET radionuclides like Gallium-68 and Copper-64 is through commercial solid-target solutions suitable for routine use.”
As the industry prepares to usher in this new era of precision health and personalized medicine, GE Healthcare is proud to offer innovative molecular imaging solutions to healthcare systems around the world.
Discovery with the Molecule Journey: Enabling Precision Health: The enablement of theranostics in prostate cancer care begins with the production of radioisotopes for use in diagnostic tracers – namely Gallium-68 PSMA-11 – which is administered to the patient, attaches to specific cancer cells, and releases radioactive emissions to provide detailed molecular information unique to each patient.
However, shortages of the generators that produce Gallium-68 historically have created serious challenges for clinicians and limited patient access. In response, GE Healthcare is proud to introduce a new Solid Target Platform for its PETtrace cyclotron which – in combination with its FASTlab 2 New Edition platform– can produce 100x the amount of Gallium compared to a generator for increased theranostics capabilities and access in prostate cancer patient care[iv].
While solid targets have been around for some time, they have traditionally been viewed as research tools and required complicated infrastructure and highly trained operators. Now, with GE Healthcare’s TRACERcenter Solutions and new PETtrace Solid Target Platform, healthcare systems can more easily access the equipment, tracers and staff training necessary to deliver a more cost-effective, personalized solution.
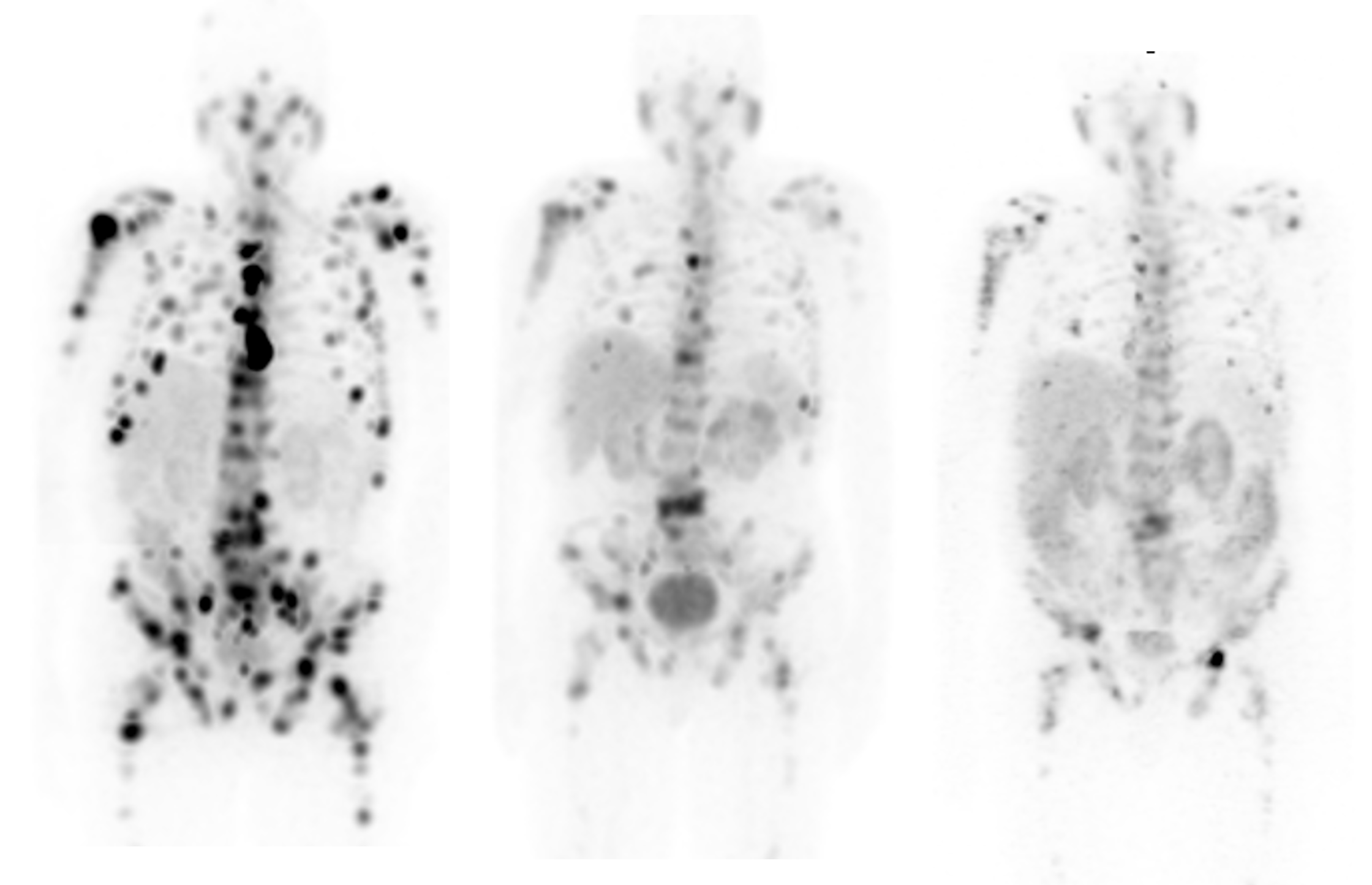
Diagnosis: Accurately Staging & Quantifying Disease: To read the emissions released by the Gallium-68 PSMA-11 tracer, the patient must be imaged using a highly sensitive PET/CT scanner. This technology provides the clinician detailed information that is used to better understand the structure and function of each patient’s tissue and disease state to help form personalized therapy recommendations. The more sensitive the PET/CT, the more accurate the images and quantification.
To this end, GE Healthcare is now shipping its Discovery MI Gen 2 premium digital PET/CT system, which provides next-level digital detection with an axial field of view (FOV) scalable up to 30 centimeters to achieve a 125 percent increase in sensitivity[v]. This helps translate to 33 percent improvement in scan times or dose amounts[vi].
These capabilities are further supported by Q.Clear, which offers up to 2x improvement in both image quality (SNR) and quantitation accuracy (SUV[vii]), and MotionFree for up to 67 percent improvement in lesion volume measurements, helping inform clinicians’ prostate cancer therapy recommendations[viii].
Additionally, this scanner includes a CT that is designed to allow TrueFidelity deep-learning image reconstruction to enable image sharpness and improved noise texture[ix]. Discovery MI Gen 2 proclaims up to a 41 percent increase in small lesion detectability[x].
Treatment: Delivering & Monitoring Targeted Therapy: With regard to therapy, the FDA recently approved Lutetium-177 PSMA-617 – an exceptional therapy for advanced prostate cancer – in March 2022. It works by binding to and delivering a small amount of radiation to prostate cancer cells anywhere in the body to help patients with advanced prostate cancer live longer and maintain quality of life[xi].
To help clinicians evaluate the success of these therapies, GE Healthcare developed its breakthrough StarGuide SPECT/CT system with 12 cutting-edge CZT detectors that not only scan patients in 3D to provide more information to clinicians but are also optimized for Theranostics procedures – including imaging this latest Lutetium-177-based prostate cancer therapy.
Compared to conventional technologies, StarGuide’s Digital Focus CZT detectors offer improved volume sensitivity and SPECT resolution[xii], which is especially valuable for imaging both peaks of Lutetium-177 emissions, which in turn helps clinicians pinpoint the size, shape, and position of lesions with exceptional accuracy. Paired with GE Healthcare’s innovative Q.Clear solution for SPECT reconstruction, the resulting images provide outstanding quantification for the diagnosis and staging of disease and monitoring of treatment.
Increasing Accuracy & Efficiency: Artificial intelligence (AI) also offers new opportunities to streamline workflows, provide accurate data, and help expedite diagnoses across care areas – all valuable offerings in today’s resource constrained healthcare environment.
That’s why GE Healthcare also offers the Xeleris V image processing solution with a collection of AI-enabled clinical applications to help simplify and enhance workflows. This includes Q.Thera AI[xiii], which is designed to leverage Q.Volumetrix MI to help clinicians automatically and accurately segment areas of interest – including AI-based kidney segmentation – for quantitation and dosimetry calculations, all with the goal to help reduce the time required for the user to process and calculate dose – enabling them to spend more time with patients.
“As we continue to see more novel, FDA cleared tracers and therapies come to market, we excited by new opportunities to adopt theranostics in every day clinical practice – enabling more personalized patient care for improved outcomes,” says Dr. Anthony Chang, founder & CEO, BAMF (Bold Advanced Medical Future) Health. “PSMA radiotherapy for prostate cancer is the most recently approved treatment, but there is great opportunity for the application of theranostics across various forms of cancer. We see excellent opportunities with our PETtrace and StarGuide systems today and look forward to a broader adoption of theranostics to achieve what we called true ‘intelligence-based precision medicine’ – all in one session.”
GE Healthcare is proud to offer clinicians unique opportunities to make personalized care decisions and treatment response assessments for the benefit of patients around the world. The company is uniquely positioned to advance these efforts as the only partner with solutions spanning from molecular imaging diagnostics, cyclotrons, chemistry synthesis, PET/CT, PET/MR, nuclear medicine, advanced digital solutions, and pharma partnerships to cover the breadth of steps from discovery to diagnosis to treatment.
For more information on GE Healthcare’s Molecular Imaging portfolio, visit gehealthcare.com or our SNMMI 2022 event overview page.
###
About GE Healthcare:
GE Healthcare is the $17.7 billion healthcare business of GE (NYSE: GE). As a leading global medical technology, pharmaceutical diagnostics and digital solutions innovator, GE Healthcare enables clinicians to make faster, more informed decisions through intelligent devices, data analytics, applications, and services, supported by its Edison intelligence platform. With over 100 years of healthcare industry experience and around 48,000 employees globally, the company operates at the center of an ecosystem working toward precision health, digitizing healthcare, helping drive productivity and improve outcomes for patients, providers, health systems and researchers around the world.
Follow us on Facebook, LinkedIn, Twitter, and Insights for the latest news, or visit our website www.gehealthcare.com for more information.
[iv] Svedjehed et al. “Demystifying solid targets: Simple and rapid distribution-scale production of [68Ga]GaCl3 and [68Ga]Ga-PSMA-11.” Nuclear Medicine and Biology. Volumes 104–105, January–February 2022, Pages 1-10. https://doi.org/10.1016/j.nucmedbio.2021.10.002
[v] Sensitivity (cps/kBq) as compared to Discovery MI 20 cm.
[vii] SNR and SUV improvement as compared to OSEM
[viii] Compared to non-processed (STATIC, no motion correction) data. As demonstrated in phantom testing using a typical and fast respiratory model, 18 mm Ge-68 spheres, and OSEM reconstruction.
[xii] StarGuide SPECT reconstruction with scatter used the system’s factory NEMA NU 1-2018 resolution protocol which uses the same method (BSREM with Clarity 3D) as its clinical bone protocol. NM/CT 870 DR and NM/CT 870 CZT SPECT reconstruction used Evolution for Bone (OSEM). NM/CT 870 DR used LEHR/LEHRS collimators and NM/CT 870 CZT used the WEHR collimator.
[xiii] CE marked. 510k pending with the FDA. Not available for sale in all regions.
Note: Radiopharmaceuticals may not be approved by ministers of health in all regions. Gallium-68 PSMA-11 and Lutetium-177 PSMA-617 are not approved in Canada.
https://www.ge.com/news/press-releases/healthcare-is-personal-ge-healthcares-total-molecular-imaging-solutions-enable





