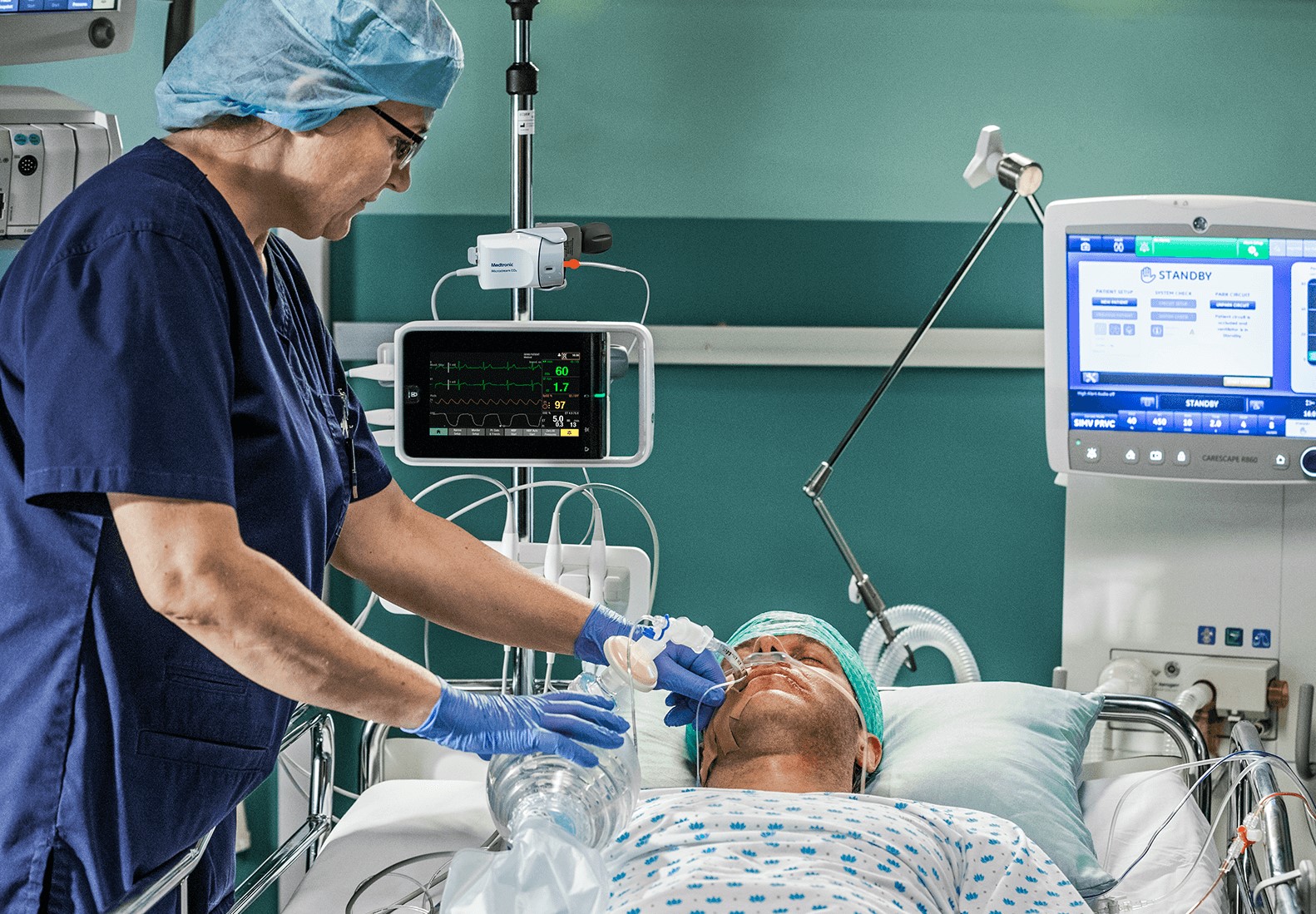
- GE Healthcare, Medtronic receive FDA 510(k) clearance and CE Mark approval on the integration of advanced INVOS™ regional oximetry and Microstream™ capnography technologies on the CARESCAPE precision monitoring platform
- Helping providers improve patient outcomes and safety, Microstream™ capnography (CO2) technology captures evolving respiratory compromise while INVOS™ regional oximetry (rSO2) technology helps clinicians predict and prevent perioperative complications quicker than traditional peripheral measurement[i],[ii]
- Integration allows for continuous monitoring access at the bedside, in transport, and networked to the electronic medical record (EMR), helping providers increase efficiency, enhance patient safety, and improve quality of care
Chicago and Dublin – June 1, 2022 – Clinicians now have added ability to personalize care with the integration of two continuous monitoring solutions from Medtronic plc (NYSE: MDT) – Microstream™ capnography (CO2) and INVOS™ regional oximetry (rSO2) technology for perioperative and ICU care on the GE Healthcare (NYSE: GE) CARESCAPE precision monitoring platform. FDA clearance and CE Mark approval of the integration of these latest technologies on the CARESCAPE platform completes the full suite of Medtronic patient monitoring technologies available on the system. The combined solution also features GE Healthcare FlexAcuity which gives clinicians around the world the ability to choose care options based on patient acuity needs and assist them in their goal of earlier detection of patient deterioration.
“Our longstanding collaboration with Medtronic allows GE Healthcare to integrate clinically advanced parameters to enable clinicians with precision monitoring for individualized care,” said Neal Sandy, general manager for Monitoring Solutions at GE Healthcare. “We are able to add these capabilities to both new and existing enterprise monitoring systems, ensuring clinicians have access to real-time, reliable patient insights that can play a critical role in care decisions and outcomes.”
Since before the inception of the CARESCAPE platform, Medtronic solutions have been integrated into GE Healthcare technologies for comprehensive patient monitoring. This partnership with Medtronic helps enable GE Healthcare’s vision to connect caregivers and their patients within an ecosystem that aims to support patient care, enable better decision making, and drive efficiencies for healthcare systems.
“Personalizing care to each patient is the standard of care. As providers, we prioritize how care is given based on individual patient acuity, so, when we’re working with a monitoring platform, it is helpful to be able to choose from different parameters to aid in capturing signs of deterioration as quickly as possible,” said Dr. Eva Masso Lago, MD, PhD, anesthesiologist with Germans Trias I. Pujol Hospital in Barcelona, Spain, who utilizes both CARESCAPE and Medtronic INVOS™ monitors to care for cardiac surgery patients. “Bringing the new INVOS™ technology into the CARESCAPE platform will add ergonomic benefits, enhance ease of use and improve workflow.”
“Medtronic and GE Healthcare are both committed to expanding access to patient care around the world,” said Frank Chan, president of the Patient Monitoring business, which is part of the Medical Surgical Portfolio at Medtronic. “This collaboration brings together our combined medical technology leadership to improve workflow, allowing clinicians more time to focus on what matters most – the patient. Together, we are empowering clinicians with actionable insights to personalize care.”
GE Healthcare and Medtronic have received CE (Conformité Européenne) Mark on the technologies included in this advanced system as well as U.S. Food and Drug Administration (FDA) 510(k) clearance for CARESCAPE software v3.2 with Microstream™ and INVOS™.
The Microstream™ capnography and INVOS™ monitoring systems should not be used as the sole basis for diagnosis or therapy and are intended only as adjunct in patient assessment.
###
About GE Healthcare:
GE Healthcare is the $17.7 billion healthcare business of GE (NYSE: GE). As a leading global medical technology, pharmaceutical diagnostics and digital solutions innovator, GE Healthcare enables clinicians to make faster, more informed decisions through intelligent devices, data analytics, applications and services, supported by its Edison intelligence platform. With over 100 years of healthcare industry experience and around 48,000 employees globally, the company operates at the center of an ecosystem working toward precision health, digitizing healthcare, helping drive productivity and improve outcomes for patients, providers, health systems and researchers around the world.
Follow us on Facebook, LinkedIn, Twitter, Instagram and Insights for the latest news, or visit our website www.gehealthcare.com for more information.
About the Patient Monitoring Operating Unit at Medtronic:
With patient safety at our core, the Patient Monitoring business develops technologies that provide clinicians with actionable insights to personalize care. Our solutions will enable clinicians to predict and prevent complications reducing costs and improving outcomes.
About Medtronic:
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 90,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic’s periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
[v] Murkin JM, Adams SJ, Novick RJ, et al. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg. 2007;104(1):51–58.
For media inquiries, please contact:
Jennifer PurdueCommunications Director
GE Healthcare
+1-267-593-9735
[email protected]
Tammy Hudson
Public Relations
Medtronic
+1-678-488-5337
Ryan Weispfenning
Investor Relations
Medtronic
+1-763-505-4626
business unit
https://www.ge.com/news/press-releases/ge-healthcare-medtronic-partnership-accelerates-global-access-to-personalized-care





