Introduction
In a recently published comparison1 of the ambient ionization techniques direct analysis in real time (DART™ 2) and DESI3, it was reported that a protonated molecule was not observed for DART, whereas the protonated molecule could be observed for DESI and DAPCI. This is an incorrect observation, resulting from the use of different experimental conditions for DART than were used for the other two techniques. Mass spectra of aspirin measured on a JEOL AccuTOF-DART™ mass spectrometer under the correct operating conditions are shown here. All assignments for the mass spectral peaks were confirmed by exact mass measurements.
Discussion
Because aspirin (acetylsalicylic acid, Figure 1) is an organic acid, it has a relatively low proton affinity. Acidic compounds tend to lose protons rather than gain them. That is, proton transfer from protonated water clusters is not favored.
Figure 1. Aspirin (acetylsalicylic acid, C9H8O4)
Organic acids tend to deprotonated to form negative ions ([M-H]–). DART analysis in negative-ion mode is the preferred approach for analyzing organic acids. Aspirin is no exception: it readily deprotonates to produce [M-H]– in negative-ion mode (Figure 2).
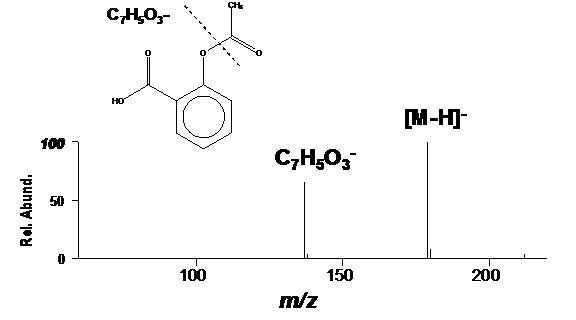
Figure 2. Negative-ion DART mass spectrum of aspirin.
If ammonium is present, aspirin will readily form an abundant [M+H]+ and a corresponding [M+NH4]+. This is shown in Figure 3 for aspirin where ammonium is present (from the headspace of a dilute aqueous ammonium hydroxide solution),
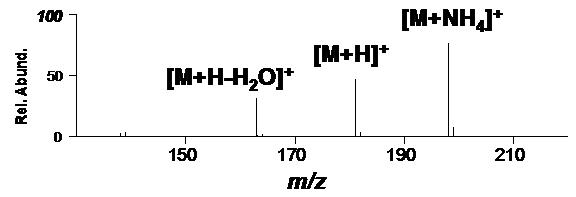
Figure 3. Positive-ion DART mass spectrum with ammonium present.
and in Figure 4 for an over-the-counter analgesic like the tablet described in reference 1. Sampling position was not critical for the DART experiment provided that one does not block the mass spectrometer atmospheric pressure ionization interface. The analgesic tablet was dangled in the DART gas stream, allowing the gas stream to graze the tablet surface to produce the spectra shown in Figure 4.
Conclusion
In reference 1, protonated aspirin was observed in the DESI and DAPCI experiments because ammonium was added. Ammonium was not present for the DART experiment and the protonated molecule was therefore not observed.
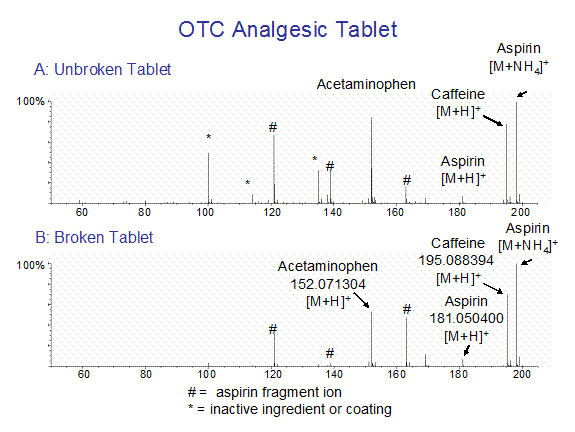
Figure 4. DART mass spectra of an over-the-counter analgesic tablet containing aspirin, caffeine, and acetaminophen.
Reference
- “The use of recently described ionisation techniques for the rapid analysis of some common drugs and samples of biological origin”, Jonathan P. Williams, Vibhuti J. Patel, Richard Holland, James H. Scrivens, Rapid Communications in Mass Spectrometry, 20(9), 2006, pp. 1447-1456. http://dx.doi.org/10.1002/rcm.2470
- “Versatile New Ion Source for the Analysis of Materials in Open Air under Ambient Conditions” Robert B. Cody, James A. Laramée, and H. Dupont Durst
Anal. Chem.; 2005; 77(8) pp 2297 – 2302; (Accelerated Article) DOI: 10.1021/ac050162j - DART™ is a trademark of JEOL USA, Inc.
- “Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization”, Zoltán Takáts, Justin M. Wiseman, Bogdan Gologan, R. Graham Cooks, Science, 2004; 306(1595),471-473 http://www.sciencemag.org/cgi/content/abstract/306/5695/471
Related web links
- www.jeolusa.com/PRODUCTS/AnalyticalInstruments/MassSpectrometers/AccuTOFDART/tabid/230/Default.aspx
- en.wikipedia.org/wiki/DART_ion_source
https://www.jeolusa.com/NEWS-EVENTS/Press-Releases/PostId/74/DART-Analysis-of-Aspirin-Correcting-a-Misapprehension





